Elements are classified broadly into two categories on the basis of properties: Metals: Iron, Zinc, Copper, Aluminium etc.
Non–metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc.
Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, and Germanium .They are called metalloids
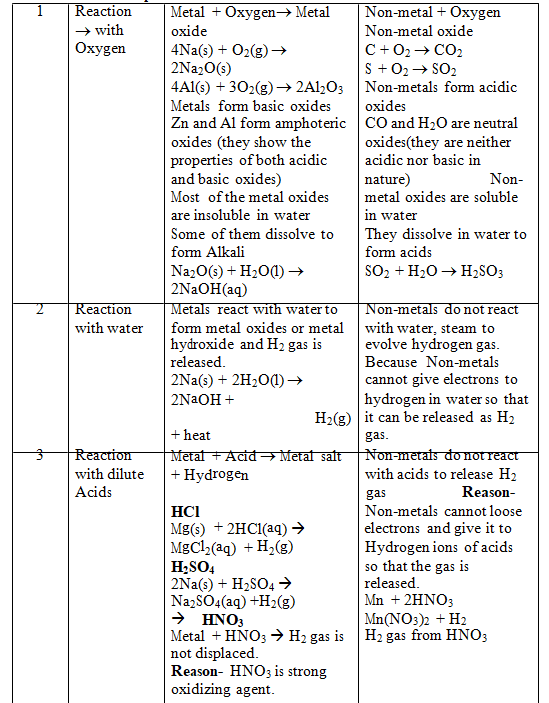
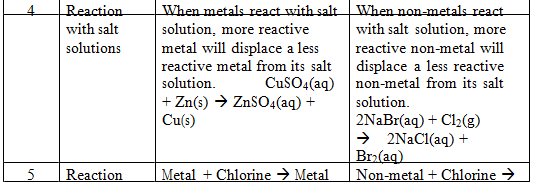
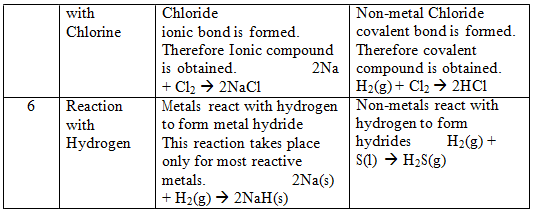
Comparison of physical and chemical properties of metals and non–metals:-

Comparison of Chemical Properties of Metals and Non-metals:–
Properties of ionic compounds
1. Physical nature:solid and hard due to strong force of attraction. (generally brittle)
2. Melting point and boiling point:have high M.P and B.P, as large amount of heat energy is required to break strong ionic attraction.
3. Solubility: soluble in water and insoluble in kerosene and pertrol.
4. Conduction of electricity:ionic compounds in solid state—–does not conduct electricity.
Reason—Ions can not move due to rigid solid structure. Ionic compounds conduct electricity in molten state.Reason– Ions can move freely since the electrostatic forces of attraction between the oppositely charged ions are overcome due to heat



METALS AND NON – METALS
FORMATIVE ASSESSMENT I
Q.PAPER
MARKS-30 TIME- 70 MINUTES
Instructions:
· Questions: 1 to 5 – 1 Mark each · Questions : 6 to 9 – 2 Marks each
· Questions: 10 to 13 – 3 Marks each · Questions 14 – 5 Marks
1) Which metal other than mercury is liquid at room temperature?
2) Why the item made of silver turn black when exposed to air?
3) Which non–metal is lustrous?
4) What is an amalgam?
5) What is the nature of oxides of metal?
6) Give reasons for the following:
a) Na, K and Ca metals form hydrides by combination with hydrogen gas, but most other metals do not.
b) Metals conduct electricity.
7) Write the equations for the reactions of: a) Iron with steam.
b) Calcium and potassium with water.
8) What is activity series? How does it help us in predicting the relative reactivities of various metals?
9) What is the difference between sodium atom and sodium ion?
10)
a) Write electron dot structure for sodium and oxygen. b) Show the formation of Na2O by electron transfer. c) What are the ions present in these compounds?
11)Write three properties of ionic compounds.
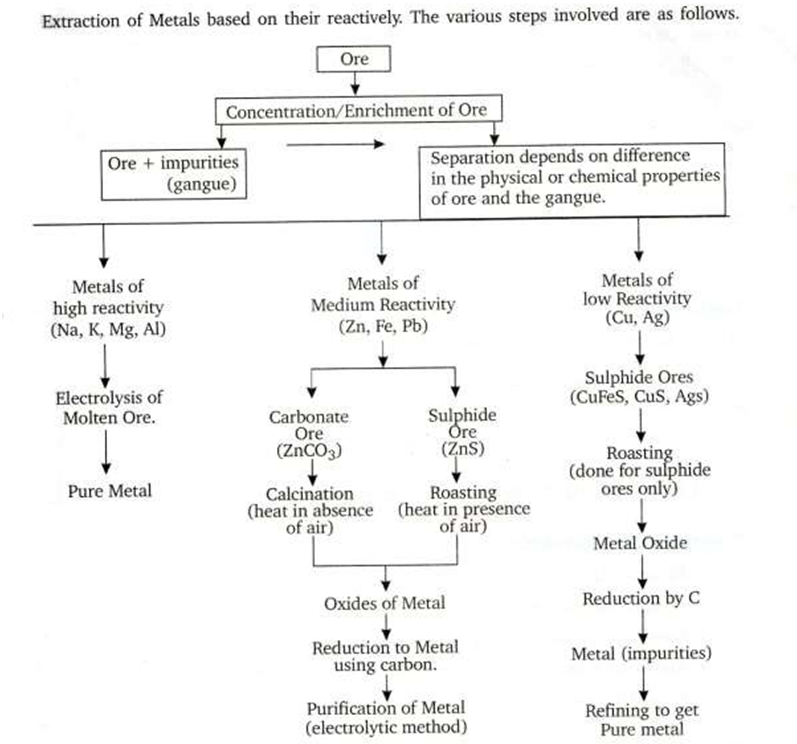
12)Explain how a metal low in the activity series can be extracted. Write suitable example.
13)Give reasons:
a) Platinum, gold and silver are used to make jewellery.
b) Sodium, potassium and lithium are stored under oil.
c) Aluminium is a highly reactive metal; still it is used to make utensils for cooking.
14)Name the following:
a) A non – metal that is a good conductor of electricity. b) A metallic oxide which cannot be reduced by coke. c) A metallic oxide which is amphoteric in nature.
d) A non – metallic oxide which is neutral. e) Principal ore of aluminium.
HOTS QUESTIONS (SOLVED / UNSOLVED)
Q.1 a) What are amphoteric oxides? Choose the amphoteric oxides from amongst the following: Na2O, ZnO, Al2O3, CO2, H2O
b) Why is it that non metals do not displace hydrogen from dilute acid?
Ans. a) The oxides which are acidic as well as basic in nature are called amphoteric oxides. ZnO and Al2O3 are amphoteric oxides.
b) Non metals can not loose electrons so that H ions become hydrogen gas. Q.2. What is anodizing? What is its use?
Ans. The process of forming thick oxide layer of aluminium oxide that makes it resistant to further corrosion.
Q.3. What is Aqua regia? What is its use?
Ans. It is a mixture of concentrated HCl and concentrated HNO3 in the ratio 3:1. It can dissolve gold and platinum.
Q.4. Give reason: Aluminium is highly reactive metal, but it is used to make utensils for cooking.
Q.5. Explain why (a) Iron articles are frequently painted. (b) Iron sheets are coated with Zinc layer.
Q.6 On adding dilute HCl acid to copper oxide powder, the solution formed is blue – green. Predict the new compound formed which imparts a blue – green colour to the solution? Write its equation.
Q.7. Name the property of metal used in the following cases- (i) Aluminium foil (ii) Meta jewellery (iii) Cable wires (iv) Bells
Q.8. How can you prove that Zinc is more reactive than Copper? Q.9. Draw and explain the electrolytic refining of impure Copper.
Q.10. Why is Aluminium extracted from Alumina by electrolytic reduction and not by reducing it with Carbon?
Q.11 Write 3 points of difference between Calcination & Roasting?
Q.12 Write 5 points of difference between Ionic compound and covalent compound. Q.13 What is thermit reaction? Give its one use.
Q.14 What is amalgam?
Q. 15 Magnesium when reacts with hot water, starts floating. Why?
FA II
METALS AND NON – METALS
ORAL QUESTIONS
1. Name the metal which is a liquid.
2. Name the non – metal which shows lustre.
3. Name the lightest metal.
4. Name the metal with highest density.
5. Name the property of the metals by virtue of which these can be beaten into sheets
6. Name the property of the metals by virtue of which these can be drawn into wires.
7. Name the material which is kept in water.
8. Name the metal used for galvanisation of iron.
9. Mercury is liquid and a good conductor of heat. How is this property utilized?
QUIZ – WHO AM I
1. I am a property of metals which appears at lower temperatures.
2. I am noble conductor of heat and electricity.
3. Though I get corroded in atmosphere but still find wide applications for making kitchen utensils.
4. I am a metal but very soft and cannot be kept in the open.
5. I am called a series and play a significant role when a metal reacts with solutions of other metal salts.
6. Scientists / Industrialists use me to extract metals profitably and economically.
7. I am a process to refine metals of high reactivity.
8. I am a process associated with wasting away of metals by the action of atmospheric gases and moisture
9. I am homogenous and not a compound though my formation least to altering the properties of metals involved.
10. We belong to the same category of elements but still combine to form molecules / compounds.

GIPHY App Key not set. Please check settings