1. Bonding in Carbon
Carbon form covalent bonds.
Formation of covalent bond : Covalent bond formation involves sharing of electrons between bonding atoms which may be either same or different.
Covalency : The number of electrons contributed by an atom for sharing is known as its covalency.
Characteristics of covalent compounds :
(i) These compounds are molecular in nature (i.e. they exist as single molecules) (ii) These are insoluble in water and soluble in benzene, kerosene and petrol etc. (iii) These compounds are poor conductor of electricity.
2. Allotropy in Carbon
The property due to which an element exists in two or more forms, which differ in their physical and some ofthe chemical properties is known as “Allotropy” and the various forms are called “Allotropes”.
∙ Carbon exists in two allotropic form (i) crystalline (ii) amorphous. The crystalline forms are diamond and graphite whereas the amorphous forms are coal, charcoal, lamp black etc.
∙ Fullerenes form another class of carbon allotropes. The first one to be identified was C 60, which has carbon atoms arranged in the shape of a football.
3. Unique Nature of Carbon
Catenation : The property of elements to form long chains or rings by self linking of their own atoms- through covalent bonds is called catenation. The extent of catenation depends upon the strength of the bonds between the atoms involved in catenation.
4. Saturated and Unsaturated Carbon Compounds
In saturated compounds the valencies of all the carbon atoms are satisfied by single bonds between them.
While in the unsaturated compounds, the valencies of all the carbon atoms are not satisfied by single bonds, thus in order to satisfy their valencies, they form double or triple bond between the carbon atoms.
5. Straight chain compounds : The compounds which contain straight chain of carbon atoms e.g. normal butane (C4H10), normal pentane (5H12) etc.
6. Branched chain compounds : Those compounds which are branched. e.g. iso-butane (C4H10), isopentane (C5H12), neopentane (C5H12) etc.
7. Closed chain compounds or Ring compounds :
Cyclic compounds are called closed chain or ring compounds e.g. cyclohexane (C6H12), cyclopentane (C5H10), cyclobutane (C4H8), cyclopropane (C3H6) etc.
8. Hydrocarbons
All those compounds which contain just carbon and hydrogen are called hydrocarbons. 9. Functional Group
The atom or group of atoms which determine the properties of a compound is known as functional group. e.g. —OH (alcohol), —CHO (aldehyde), > C = C < (alkene), — C—C — (alkyne) etc.
10. Homologous Series
A series of compounds in which the same functional group substitutes hydrogen in a carbon chain is called a homologous series.
e.g. CH3C1 and C2H5C1 differ by a —CH2 unit.
11. Nomenclature
Chemists developed a set of rules, for naming organic compounds based on their structures which is known as IUPAC rules.
The IUPAC name of an organic compounds consists of three parts.
Prefix – word root – Suffix
Word Root : A word root indicates the nature of basic carbon skeleton.
In case a functional group is present, it is indicated in the name of the compound with either as a prefix or as a suffix.
While adding the suffix to the word root the terminal „e‟ of carbon chain is removed If the carbon chain is unsaturated then the final `ane‟ in the name of the carbon chain is substituted by „en& or yne‟ respectively for double and triple bonds.
(i) Combustion : Carbon compound undergo combustion reaction to produce CO2 and H20 with the evolution of heat and light.
CH4 +O2 > CO2 + 2O + heat and light
(ii) Oxidation :

The substance which are used for oxidation are known as oxidising agent.
e.g alkaline KMnO4, acidified K2Cr2O7.
(iii) Addition reaction :
Unsaturated hydrocarbons (alkenes and alkynes undergo addition reaction in presence of catalysts e.g.

4 | P a g e
(iv) Substitution reaction : Saturated hydrocarbons give substitution reaction e.g. methane in presence of sunlight undergo chlorination.
13. Some Important Carbon Compounds
Alcohols : Compounds containing -OH group attached to a carbon atom are known as alcohols. Example : Ethanol (C2H5OH) : commonly known as alcohol.
Properties of ethanol :
1. Reaction with sodium : Due to its weakly acidic nature, ethanol reacts with sodium to librate H2 gas.
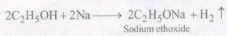
2. Reaction with conc : H2SO4 :

Alcohol as a fuel : Alcohol (ethanol) is added to petrol upto 20% and the mixture is called “gasol”.
Harmful Effects of Drinking Alcohol :
If the alcohol used for drinking purposes contains some methyl alcohol (CH3OH) as impurity then it may cause serious poisoning and loss of eye sight.
It is habit forming and damages liver if taken regularly in large quantities. Ethanoic Acid (Acetic Acid) CH3COOH:
Ethanoic acid, commercially known as acetic acid belongs to a group of acids called carboxylic acid.
Chemical properties :
(i) Reaction with a base :
2CH3COOH + 2NaOH → 2CH3COONaH2O
(ii) Reaction with carbonates and bicarbonates :
2CH3COOH + Na2CO3 → 2CH3COONa + CO2 + H2O
5 | P a g e
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
(iii) Reaction with alcohol : (Esterification)

Esters react is the presence of an acid or a. base to give back the alcohol and carboxylic acid this reaction is known as saponification.
14. Soaps and Detergents :
Soaps : Soaps are sodium or potassium salts of long chain acid carboxylic acids. Detergent : They are ammonium or sulphurate salts of long chain carboxylic acids.

GIPHY App Key not set. Please check settings